Which Describes an Atom of Plutonium
94 the most stable isotope of this element. Electron configuration Rn7s2 5f6.

Plutonium Electron Configuration Symbol Atomic Number Atomic Mass Oxidation States Standard State Group Block Year Discovered
The chemical symbol for Plutonium is Pu.

. Pu e Pu H Affinity kJmol. Plutonium-239 is an isotope of plutonium. Neutrons inside its nucleus.
All radiation can be characterized. 244 94 150. DIt has an atomic number of 244.
Since plutonium has an atomic number equal to 94 you can say that plutonium-244 contains. Which statement most likely describes plutonium. An atom of Plutonium in the gas phase for example gives off energy when it gains an electron to form an ion of Plutonium.
When this occurs different pieces of the original atom remain including a substance called plutonium. U-238 becomes thorium-234 for. The nucleus consists of 94 protons red and 150 neutrons orange.
In special circumstances that usually involve high amounts of energy uranium can be broken. Answer 1 of 3. CIt does not possess all the properties of a larger quantity of plutonium.
Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons although uranium-235 is also used for that purpose. Which of the following describes the relationship between frequency and wavelength for electromagnetic radiation. Which statement most likely describes plutonium.
The chemical symbol for Plutonium is Pu. It must no longer be a form of matter. AIt can be divided into smaller particles that retain all the properties of plutonium.
Describe how this process affects the proton number and the nucleon number. 6 e I Add To Classified 2 Mark s. AIt can be divided into smaller particles that retain all the properties of plutonium.
Which describes an atom of plutonium. When this occurs different pieces of the original atom remain including a substance called plutonium. An atom of plutonium-239 he most common isotope contain 94 protons 94 electrons and 145 neutrons.
BIt cannot be divided into smaller particles that retain all the properties of plutonium. Which nucleus needs the largest number of electrons to form a neutral atom. 94 electrons white successively occupy available electron shells rings.
Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors along with uranium-235 and uranium-233. Oxidation States 6 5 4 3. C It does not possess all the properties of a larger quantity of plutonium.
Which describes an atom of plutonium. Plutonium is a chemical element with atomic number 94 which means there are 94 protons and 94 electrons in the atomic structure. Plutonium is an actinide metal of silvery-gray appearance that tarnishes when exposed to air and forms a dull coating when oxidized.
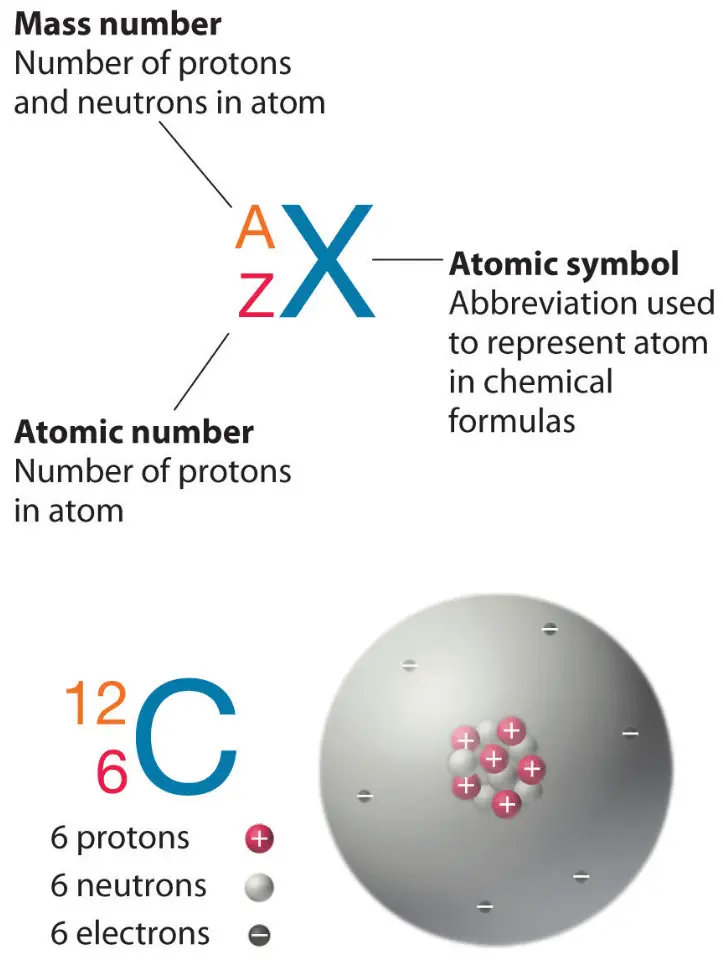
244064 244. This equation describes the process. Diagram of the nuclear composition electron configuration chemical data and valence outer electron orbitals of an atom of plutonium-244 atomic number.
Uranium is a type of atom. Typically that means that the nucleus will shed an alpha particle thereby becoming more stable. This is the case because the mass number of a nuclide is calculating by adding the number of protons which is given by the atomic number and the number of neutrons.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. Electron affinity is one of the most important parameters that guide chemical reactivity. Every nucleus heavier than 208 daltons is radioactive.
B It cannot be divided into smaller particles that retain all the properties of plutonium. What is Plutonium. Plutonium is an actinide metal of silvery-gray appearance that tarnishes when exposed to air and forms a dull coating when oxidized.
DIt has an atomic number of 244. What was the major difference between Daltons model of the atom and the plum pudding model of the atom. Which combination of protons and neutrons is possible for this form of plutonium.
Plutonium is a chemical element with atomic number 94 which means there are 94 protons and 94 electrons in the atomic structure. It will be different than but have the same properties as uranium. It will still be uranium just smaller pieces of uranium.
For other isotopes the number of neutrons is differentNumber of neutrons Mass number of an. It must be a different kind of atom. Plutonium-239 has a half.

Plutonium Periodic Table And Atomic Properties
Plutonium Periodic Table And Atomic Properties

The Crystal Structure Of A Plutonium The A Phase The Equilibrium Phase Download Scientific Diagram
0 Response to "Which Describes an Atom of Plutonium"
Post a Comment